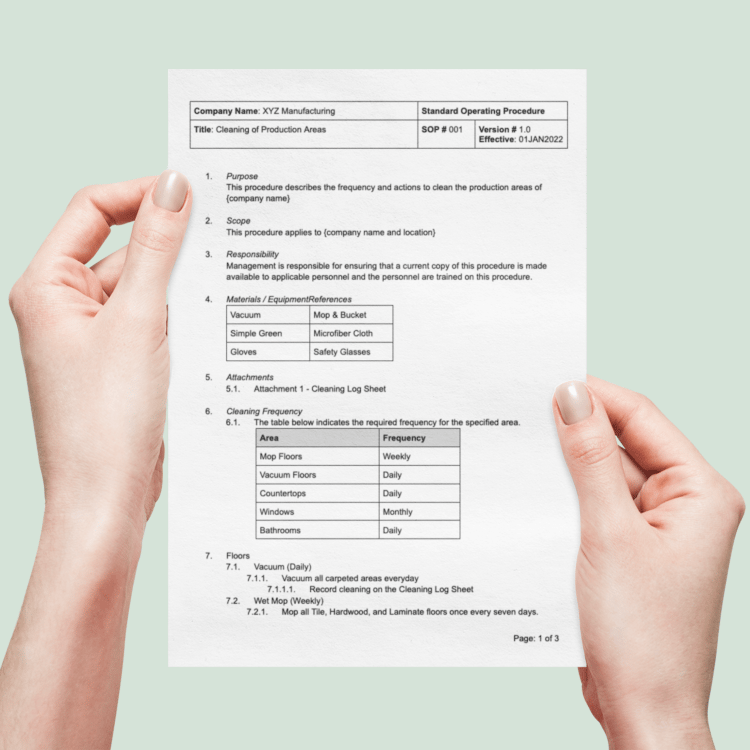
The Equipment & Facilities Pack: GMP Templates for Qualification, Calibration & Maintenance
Are you responsible for qualifying a new facility or bringing critical manufacturing equipment online under a tight deadline? The process of authoring validation protocols, establishing robust maintenance programs, and ensuring your facility is in a constant state of control is a monumental task that requires deep expertise and meticulous documentation.
The Equipment & Facilities Pack is your targeted solution to build a compliant and audit-ready infrastructure. This expert-authored bundle provides a complete framework for your engineering, facilities, and validation teams to manage your physical plant and equipment in full compliance with cGMP requirements. Stop creating essential procedures from scratch and implement a proven system for equipment and facility control.
Key Benefits:
- Streamline Qualification: Use a structured, comprehensive template for your Equipment Qualification (IQ, OQ, PQ) protocols to accelerate validation timelines and avoid common documentation gaps.
- Establish Robust Maintenance Programs: Implement detailed procedures for your Calibration and Preventive Maintenance programs to ensure equipment reliability, prevent costly downtime, and maintain compliance.
- Maintain a State of Control: Use detailed SOPs, forms, and logs for facility cleaning, environmental monitoring, and pest control to ensure your manufacturing environment is always audit-ready.
- Improve Documentation Practices: Capture all critical data with professionally designed forms and logs for equipment use, cleaning, calibration, and maintenance work orders.
What's Included in the 11 Document Pack?
This bundle provides all the necessary SOPs, Forms, and Logs to establish control over your cGMP facility and equipment, including:
- SOP-FEQ-001: Equipment Qualification (IQ, OQ, PQ)
- SOP-FEQ-002: Calibration Program
- SOP-FEQ-003: Preventive Maintenance Program
- SOP-FEQ-004: Equipment Cleaning and Use Logs
- SOP-FEQ-005: Facility Cleaning and Environmental Monitoring
- SOP-FEQ-006: Pest Control Program
- FORM-FEQ-001: Equipment Qualification Protocol Template
- FORM-FEQ-002: Calibration Record
- FORM-FEQ-003: Maintenance Work Order
- LOG-FEQ-001: Equipment Use and Cleaning Log
- LOG-FEQ-002: Room Cleaning Log
Who is this for?
This package is the perfect solution for Validation Engineers, Facilities Managers, and Quality professionals at biotech startups who are tasked with establishing a compliant GMP facility, qualifying new equipment, or preparing for an upcoming facility audit.
Important Disclaimer: Your Role in Implementation
This documentation pack provides a comprehensive set of templates designed to serve as a strong foundation. It is not a ready-to-use, off-the-shelf system. Each template must be reviewed and updated by your organization to accurately reflect your specific processes and equipment. While these documents are authored based on deep industry experience and cGMP principles, you are ultimately responsible for ensuring that your final, implemented procedures comply with all applicable regulations. We do not guarantee that these templates are "audit-proof" as-is.