Atomic Theory and Atomic Structure ( IGCSE course)
On Sale
£3.00
£3.00
This lesson covers an introduction to atomic theory. In includes a Power Point lesson covered the topics outline below. It also includes a revision or homework page (with answer sheet). A ‘Hot Seat Quiz’ that includes the key terms covered in the lesson and a printable of the key figures in Atomic Theory.
The Power Point lesson covers the key terms:
• Atomic theory
• Atoms
• Atomic structure
• Protons
• Neutrons
• Electrons
• Atomic Mass Unit
This is followed by a section covering describing atoms:
• Electron configuration
• Mass Number (Nucleon number)
• Atomic number (proton number)
The last section covers briefly
• Isotopes
• Relative Mass Unit
• Relative Formula Mass
This lesson cover the section C3 of the iGCSE unit 0653 combined science (some sections regarding the periodic table will be included in a separate section).
C3. Atoms, elements and compounds
3.3 Atomic structure and the Periodic Table
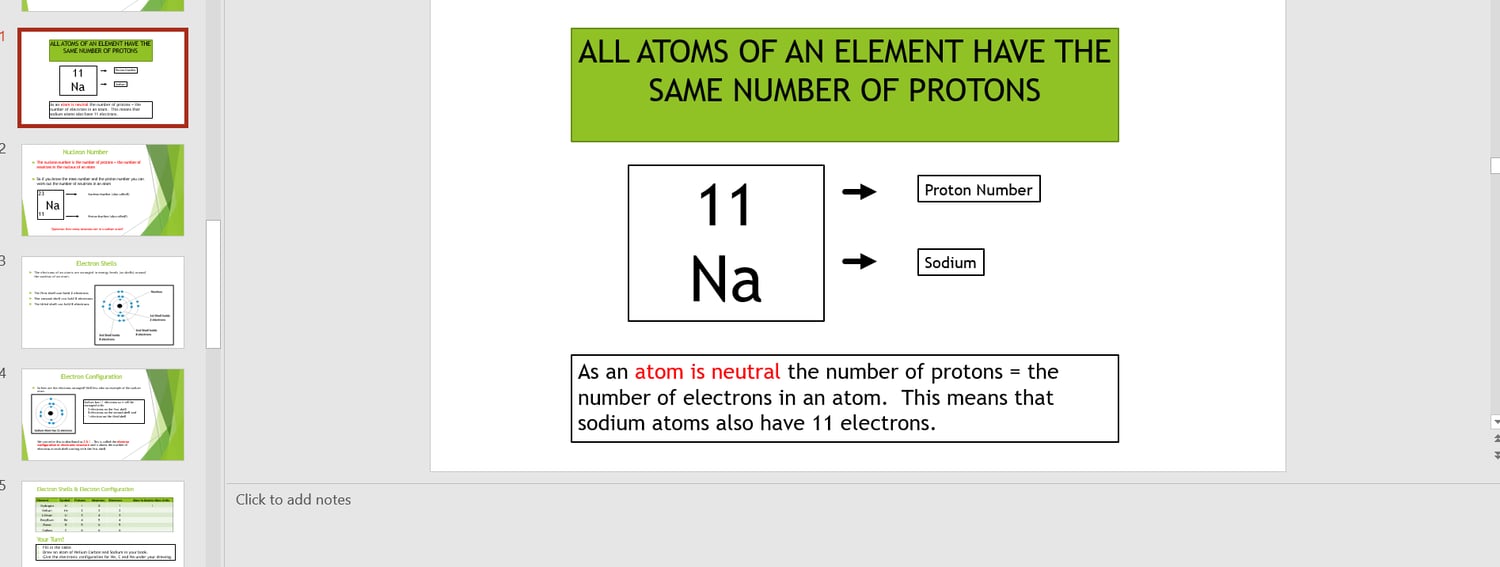
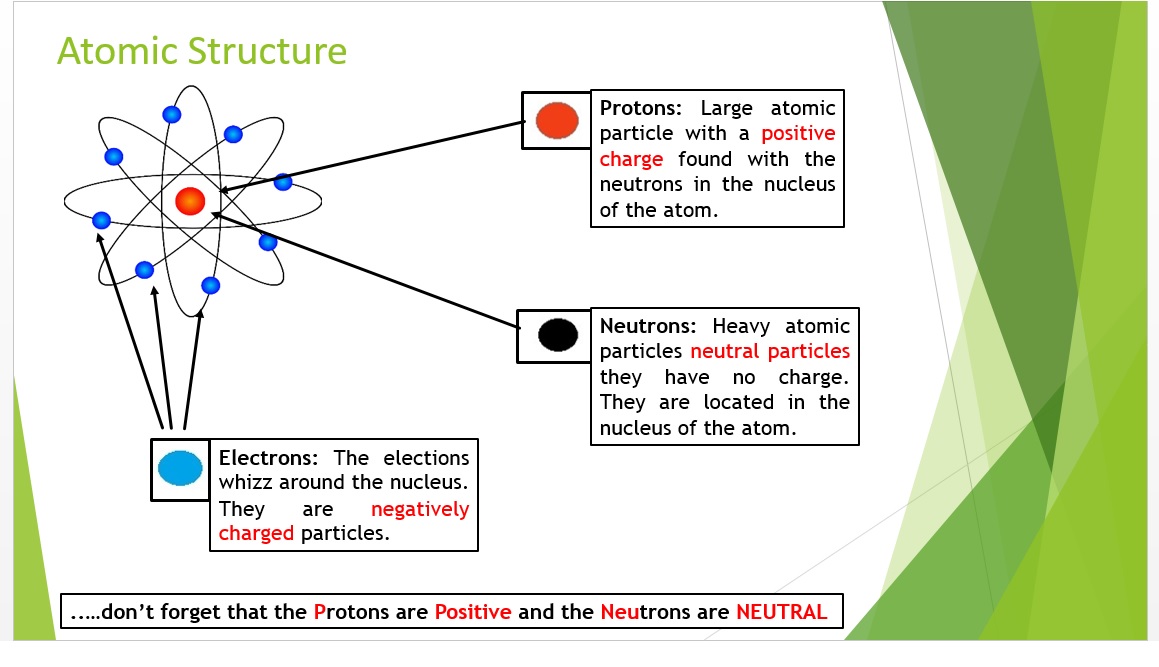
1 Describe the structure of an atom in terms of electrons and a nucleus containing protons and neutrons.
2 Describe the build-up of electrons in ‘shells’ and understand the significance of the noble gas electronic structures and of valency electrons (reference to noble gases not included - this will be in periodic table lesson)
3 State the relative charges and approximate relative masses of protons, neutrons and electrons.
4 Define atomic (proton) number and mass (nucleon) number.
5 Use proton number and the simple structure of atoms to explain the basis of the Periodic Table (see section C9), with special reference to the elements with proton numbers 1 to 20. (not yet included in this section)
The Power Point lesson covers the key terms:
• Atomic theory
• Atoms
• Atomic structure
• Protons
• Neutrons
• Electrons
• Atomic Mass Unit
This is followed by a section covering describing atoms:
• Electron configuration
• Mass Number (Nucleon number)
• Atomic number (proton number)
The last section covers briefly
• Isotopes
• Relative Mass Unit
• Relative Formula Mass
This lesson cover the section C3 of the iGCSE unit 0653 combined science (some sections regarding the periodic table will be included in a separate section).
C3. Atoms, elements and compounds
3.3 Atomic structure and the Periodic Table
1 Describe the structure of an atom in terms of electrons and a nucleus containing protons and neutrons.
2 Describe the build-up of electrons in ‘shells’ and understand the significance of the noble gas electronic structures and of valency electrons (reference to noble gases not included - this will be in periodic table lesson)
3 State the relative charges and approximate relative masses of protons, neutrons and electrons.
4 Define atomic (proton) number and mass (nucleon) number.
5 Use proton number and the simple structure of atoms to explain the basis of the Periodic Table (see section C9), with special reference to the elements with proton numbers 1 to 20. (not yet included in this section)