Upon studying this course students will be able to among many other points :
Draw and interpret the displayed formula of a molecule to show all the atoms and all the bonds
Write and interpret general formulae of compounds in the same homologous series, limited to: (a) alkanes,CnH2n+2 (b) alkenes, CnH2n (c) alcohols,CnH2n+1OH (d) carboxylic acids, CnH2n+1COOH
Identify a functional group as an atom or group of atoms that determine the chemical properties of a homologous series
State that a structural formula is an unambiguous description of the way the atoms in a molecule are arranged, including CH2=CH2, CH3CH2OH, CH3COOCH3
Define structural isomers as compounds with the same molecular formula, but different structural formulae, including C4H10 as CH3CH2CH2CH3 and CH3CH(CH3)CH3 and C4H8 as CH3CH2CH=CH2 and CH3CH=CHCH3
State that a homologous series is a family of similar compounds with similar chemical properties due to the presence of the same functional group
State that a saturated compound has molecules in which all carbon–carbon bonds are single bonds
State that an unsaturated compound has molecules in which one or more carbon–carbon bonds are not single bonds
Name and draw the displayed formulae of: (a) methane and ethane (b) ethene (c) ethanol (d) ethanoic acid (e) the products of the reactions stated in sections 11.4–11.7
Name the fossil fuels: coal, natural gas and petroleum
Name methane as the main constituent of natural gas
State that hydrocarbons are compounds that contain hydrogen and carbon only
State that petroleum is a mixture of hydrocarbons
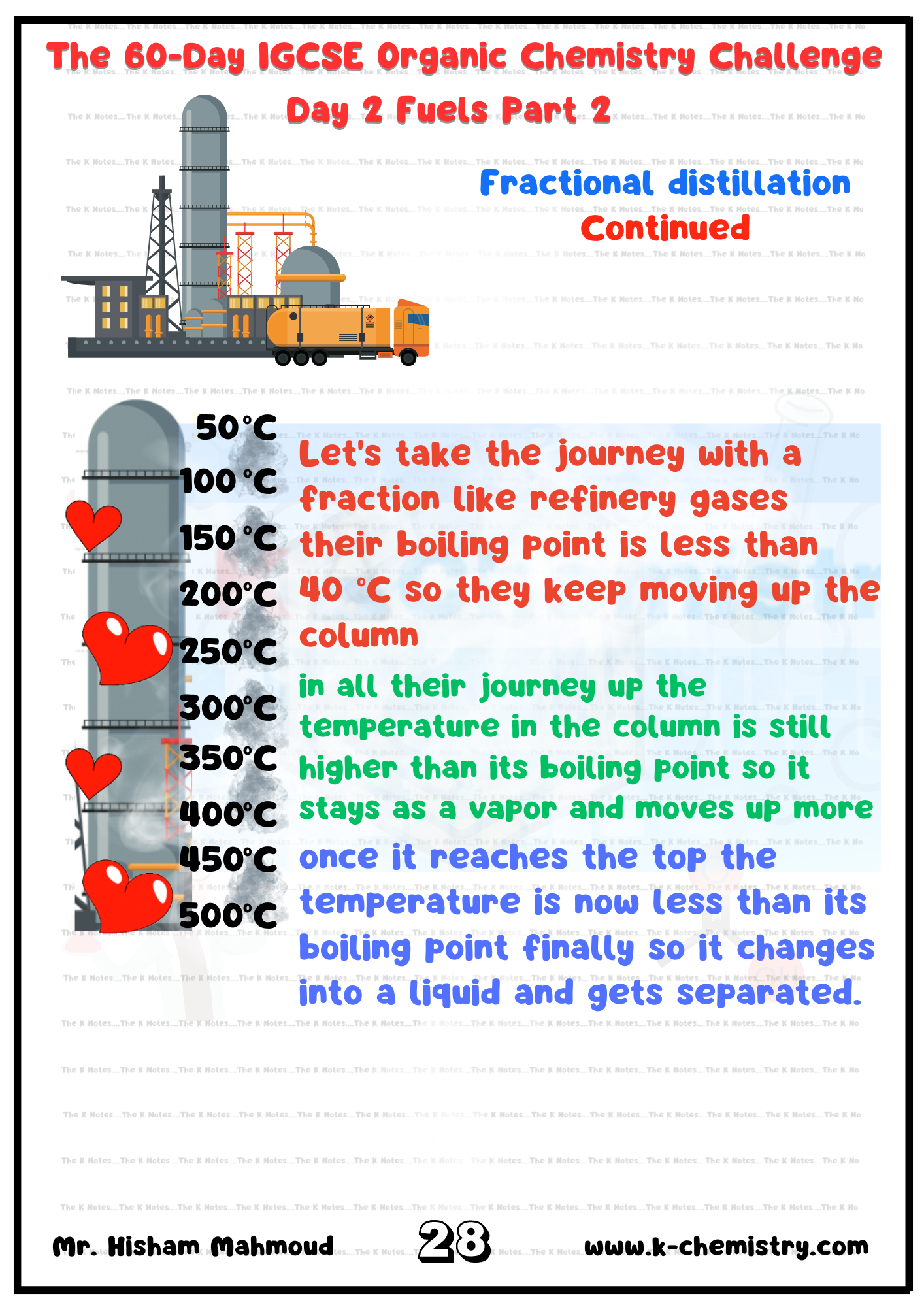
Describe the separation of petroleum into useful fractions by fractional distillation
Describe how the properties of fractions obtained from petroleum change from the bottom to the top of the fractionating column, limited to: (a) decreasing chain length (b) higher volatility (c) lower boiling points (d) lower viscosity
Name the uses of the fractions as: (a) refinery gas fraction for gas used in heating and cooking (b) gasoline / petrol fraction for fuel used in cars (c) naphtha fraction as a chemical feedstock (d) kerosene / paraffin fraction for jet fuel (e) diesel oil / gas oil fraction for fuel used in diesel engines (f) fuel oil fraction for fuel used in ships and home heating systems (g) lubricating oil fraction for lubricants, waxes and polishes (h) bitumen fraction for making roads
State that the bonding in alkanes is single covalent and that alkanes are saturated hydrocarbons
Describe the properties of alkanes as being generally unreactive, except in terms of combustion and substitution by chlorine
State that the bonding in alkenes includes a double carbon–carbon covalent bond and that alkenes are unsaturated hydrocarbons
Describe the manufacture of alkenes and hydrogen by the cracking of larger alkane molecules using a high temperature and a catalyst
Describe the reasons for the cracking of larger alkane molecules
Describe the test to distinguish between saturated and unsaturated hydrocarbons by their reaction with aqueous bromine
State that in a substitution reaction one atom or group of atoms is replaced by another atom or group of atoms